Our Services

Medical Device Regulatory Experts Supporting SMEs and Biopharma
Peritus Medical Device Group has the vital knowledge & expertise to support any aspect of Medtech Regulations, QA/RA Compliance, and Software QA where we are a leading provider.
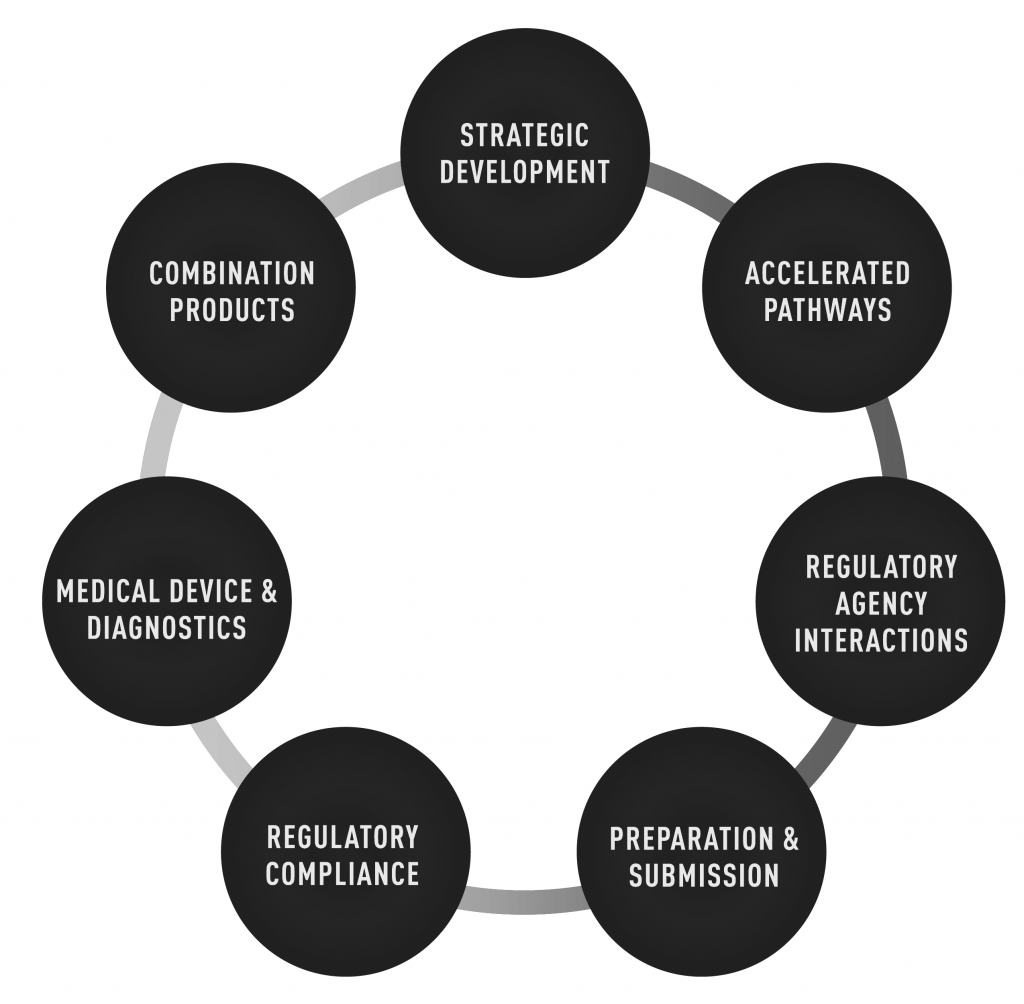
Regulatory Expertise in Development, Submission & In-market
Well designed regulatory strategies are pivotal for product development, timing for regulatory applications, discussions with approval bodies and in-market support.


Safety Toxicology, Nonclinical, Pharmacology, CMC and Pathology
Peritus has nonclinical toxicology / safety and CMC specialists - adding significant value to projects with pharmaceutical & biotechnology companies, CROs, SMEs, spin-outs and academia.

Clinical Research - Strategy, Design, Management & Reporting
Expert clinical research group which is able to support, design, manage and report on product development from phase I onwards. Excellent experience with the Notified Bodies, and regulatory authorities (FDA, MRHA, BSi etc).

Market Access / Health Economics Expertise for UK & Europe
Our expert Market Access / HEOR team helps life science and medtech companies plan and practically implement market access pathways in UK/EU.
