Medical Device Safety & Biocompatibility
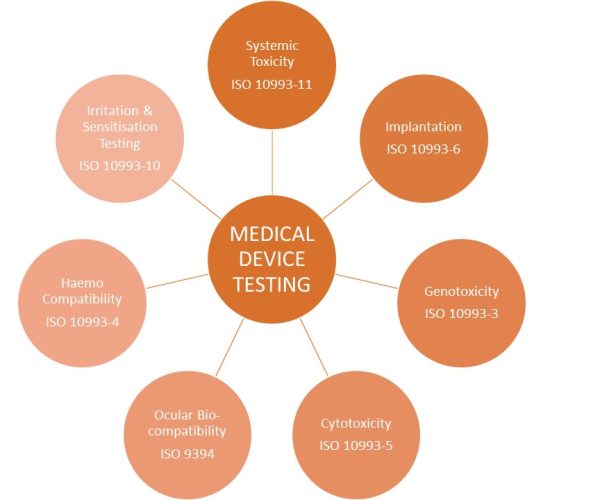
Biological safety testing for medical devices depends on classification and intended application – this determines the biocompatibility programme in line with the regulatory requirements – this is often ISO 10993 guidance which outlines the required biological evaluation required for assessing medical device safety.
Peritus can help clients in numerous ways to determine the safety and biocompatibility of their medical devices:
Determination of medical device classification and regulatory pathway advise
Medical device materials characterisation
Undertake toxicology risk/safety margin assessments as required
Compliance to regulatory requirements – gap analysis, reporting
Design, place and monitor essential studies
Please contact us for support and questions in any non-clinical safety toxicology and CMC related to products and medical devices.
