Toxicology Design, Monitoring & Reporting Services
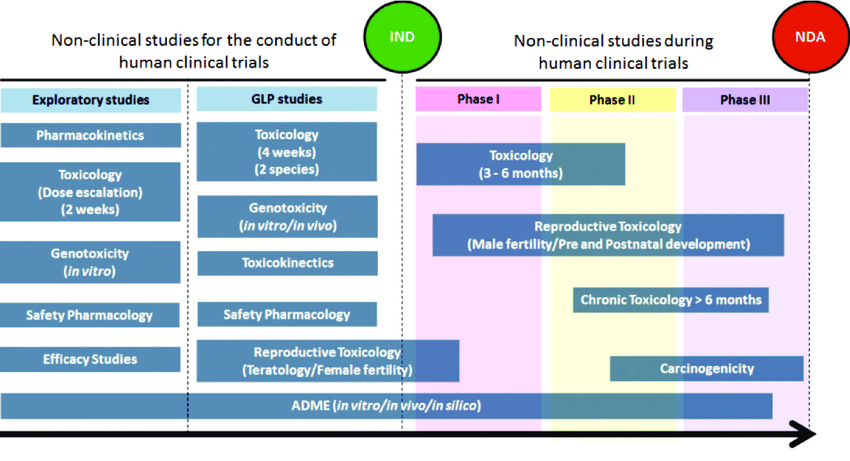
Well designed nonclinical packages are pivotal for product development, timing for regulatory applications, discussions with approval bodies. We help clients throughout this development pathway both in terms of design and execution. With strong relationships with CROs and with novel technologies, our team can help you place studies, provide independent expert monitoring and review/reporting results. Services offered:
Study Design
Intermediate Reporting
Study Monitoring Services
Quality Control
Report Review & Evaluation
Project Management of Non-clinical Packages
Please contact us for support and questions in any non-clinical safety toxicology and CMC related to products and medical devices.
